Lithium ion batteries for electric vehicles
The Department of Energy (DOE) announced Aug. 2 that a team of engineers at Washington University in St. Louis will receive $2 million to design a battery management system for lithium-ion batteries that will guarantee their longevity, safety and performance. This is a particularly challenging project because the electrochemical reactions inside the battery are not easily captured in mathematical form.
The project is one of 12 that won funding from the DOE’s Advanced Research Projects Agency-Energy (ARPA-E) under the new AMPED program that focuses on innovations in battery management and storage to advance electric vehicle technologies and to help improve the efficiency and reliability of the electrical grid.
“This latest round of ARPA-E projects seek to address the remaining challenges in energy storage technologies, which could revolutionize the way Americans store and use energy in electric vehicles, the grid and beyond, while also potentially improving the access to energy for the U.S. military at forward operating bases in remote areas,” says Secretary of Energy Steven Chu.
“These cutting-edge projects could transform our energy infrastructure, dramatically reduce our reliance on imported oil and increase American energy security,” Chu says.
“This initiative is part of a broader effort to strengthen the university’s expertise in energy-related technologies,” says Pratim Biswas, PhD, chair of the Department of Energy, Environmental & Chemical Engineering in the School of Engineering & Applied Science.
“While this grant targets car batteries,” he says, “the technology is also directly applicable to intermittent sources of energy such as solar that produce energy that may need to be buffered rather than plugged directly into electrical grid.”
The department has also recently won a large grant in solar technology and plans to launch an effort called Solar Energy and Energy Storage, or SEES.
The AMPED award goes to the Modeling, Analysis and Process-control Laboratory for Electrochemical systems (MAPLE) in the Department of Energy, Environmental & Chemical Engineering, led by Venkat Subramanian, PhD, associate professor.
“I want to give credit to my doctoral students,” Subramanian says. “Without their efforts, we wouldn’t have been able to submit a proposal. The solicitation came and we had two weeks to respond after the team was formed, and then we got a review and we had to respond over the weekend.”
In addition to Subramanian, the team includes doctoral students Venkatasailanathan Ramadesigan, Paul Northrop, Sumitava De, Bharatkumar Suthar and Matthew Lawder.
Lithium-ion batteries
Lithium-ion batteries are what are called secondary cells, because the electrochemical reactions that create a current are reversible and the battery can be recharged. The more familiar primary cells, in contrast are used once and thrown away.
The lithium is stored in metallic (uncharged) form inside the particles of a graphic electrode, explains Subramanian. During discharge the lithium comes to the electrode’s surface, where it is ionized, creating a current that travels to the cathode. At the cathode, typically a lithium-based alloy, the ions are neutralized and enter electrode particles as metallic lithium.
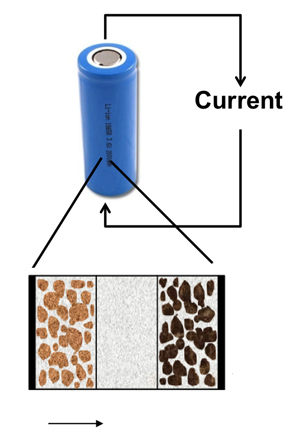
The battery is recharged by forcing a current to flow in the opposite direction, moving the lithium back into the anode.
Lithium-ion batteries hold great promise for applications such as electric vehicles because they have high energy density (energy stored per unit volume) and lose charge very slowly when not in use.
No battery is perfect and lithium-ion batteries, like all batteries, have drawbacks. If the batteries are charged too fast, they can heat up and may explode. To avoid catastrophic failure, manufacturers overdesign the batteries and use only part of their energy capacity per cycle, Subramanian says.
“The goal of the AMPED program is to push the current technology to 100-percent efficiency, while making sure battery lifetime is not compromised,” Subramanian says. This would ultimately reduce the weight of the car and improve its energy efficiency.
Revving up the models
“If you can predict what will happen inside the battery, you can push the battery to do more per cycle,” Subramanian says. “Currently empirical (experience based) models that have no predictive capability are used to manage the batteries. This is why manufacturers over-stack the material; they have no idea what’s happening inside.”
There are physics-based models of lithium-ion batteries but they are computationally intensive and can’t be solved in real time by the usual methods.
This is where the MAPLE lab comes in. The engineers plan to use a class of simulation techniques called spectral methods aided by mathematical analysis to solve a physics-based model’s differential equations. Spectral methods should allow them to cut down on the model’s computational demands so that it runs faster.
The Battery Management System (BMS), MAPLE lab develop will keep the battery operating optimally, enabling maximum utilization of energy at all times.
“In general,” Subramanian says, “people write mathematical models and then plug them into commercial software to solve them. We relish solving the models ourselves to see if we can find more elegant ways to do it. That’s the overarching theme of our work.
“We are also interested in re-examining predictive models of importance for medicine, such as those used in medical imaging, to see if we can solve them faster but with the same accuracy so that they can be used in real-time sensing and control,” he says.
Modeled on the Defense Advanced Projects Agency (DARPA), famous for its daring funding decisions, ARPA-E was launched in 2009 to seek out breakthrough technologies that are too risky for private-sector investment but have the potential to energy technology, form the foundation for entirely new industries, and have large commercial impacts.


